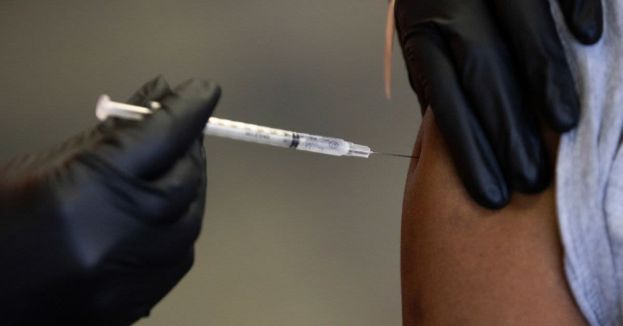
The vaccine is intended for individuals aged 18 and above who are at risk of exposure to the virus.
Chikungunya, a virus transmitted through mosquito bites, is categorized similarly to dengue or Zika, according to Dr. Marc Siegel, a clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor. "This virus is in a similar category as dengue or Zika and is carried by the same mosquitoes," he stated.
November 12, 2023
The FDA has labeled chikungunya as an "emerging global health threat," with over five million cases reported in the last 15 years. "Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions," said Peter Marks, M.D., PhD, director of the FDA’s Center for Biologics Evaluation and Research. He further added, "Today’s approval addresses an unmet medical need and is an important advancement in the prevention of a potentially debilitating disease with limited treatment options."
WATCH: BRITAIN'S HEAVIEST MAN HAS PASSED AWAY AT 33![]()
The safety of the vaccine was evaluated in clinical trials involving 3,500 adults before the FDA's approval. The most commonly reported side effects included headache, muscle pain, fatigue, joint pain, nausea, fever, and tenderness at the injection site. A small percentage of recipients (1.6%) experienced adverse reactions, with two requiring hospitalization, according to the FDA’s release.
PASTOR DODGES BULLET MID-SERMON AFTER MAN "SEES SPIRITS" AND OPENS FIRE![]()
The vaccine's efficacy was assessed in a separate study, based on the immune response data of 266 adult participants. Nearly all participants demonstrated protective antibody levels.
The Centers for Disease Control and Prevention (CDC) states that the most common symptoms of the virus are fever and joint pain, with some individuals also experiencing headache, muscle pain, joint swelling, or rash. Symptoms usually appear within three to seven days after transmission. While most people recover within a week, in rare cases, the virus can cause severe and long-lasting joint pain.
WATCH: AT WHAT POINT WE WILL TAKE THEM SERIOUSLY?![]()
The CDC identifies older adults, newborns infected at birth, and individuals with heart disease, diabetes, or high blood pressure as those at the highest risk for adverse health effects. Deaths from the virus are extremely rare.
The chikungunya virus is endemic in Africa, Southeast Asia, and parts of the Americas, according to the FDA. Prior to 2013, cases were primarily documented in Africa, Asia, Europe, and the Indian and Pacific Oceans. However, the first local cases were reported in Caribbean countries in late 2013, leading to the virus spreading throughout the Americas.
For those exposed to the virus and exhibiting symptoms, a blood test can confirm the presence of chikungunya or other similar viruses. The CDC recommends that infected individuals rest, stay hydrated, and take over-the-counter medications to alleviate and reduce fever.
MAN'S UNEXPECTED CONFESSION ON SIDE OF OKLAHOMA HIGHWAY SHAKES UP 5-YEAR-OLD COLD CASE![]()
Dr. Siegel described the vaccine as "safe and effective," containing a live weakened version of the virus. "That means it is not intended for [the] immunocompromised, but it is useful for those at risk of severe cases of chikungunya," he told Fox News Digital. He also mentioned that the vaccine is being fast-tracked, suggesting that it should be administered to those most at risk first, while monitoring post-marketing studies over the next year.



 Discover alternative ideas that will make you think
Discover alternative ideas that will make you think Engage in mind bending debate
Engage in mind bending debate Earn points, rise in rank, have fun
Earn points, rise in rank, have fun


