Scott Gottlieb, who headed the FDA under former President Donald Trump and currently sits on the board of directors at Pfizer, signaled that the emergency use approval process for vaccinating young children could be done in a matter of weeks. Gottlieb announced that the pharmaceutical giant is expected to file the paperwork with the federal government requesting authorization to vaccinate kids as early as September.
'In a best-case scenario, given that timeline they've just laid out, you could potentially have a vaccine available to children aged 5 to 11 by Halloween,' Gottlieb told CBS’s Face the Nation.
'If everything goes well, the Pfizer data package is in order, and the FDA ultimately makes a positive determination, I have confidence in Pfizer in terms of the data that they've collected.
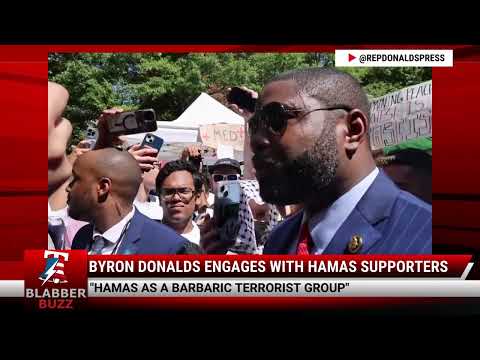
WATCH: BYRON DONALDS ENGAGES WITH HAMAS SUPPORTERS![]()
The vaccine has already been granted emergency use approval for children between the ages of 12 and 15.
Children are far less likely to contract severe cases of COVID-19. In states reporting pediatric cases, children accounted for fewer than one-quarter of 1 percent of all COVID-19 deaths, according to National Public Radio.
THE UNSEEN VICTIMS OF WAR: HOW RUSSIA'S INVASION LEFT THOUSANDS OF DISABLED UKRAINIANS IN PERIL![]()
Seven states have reported no child deaths, while other states reported 0-0.03 percent of all COVID cases in children resulting in deaths.
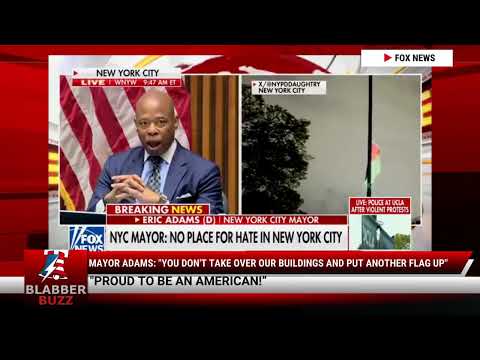
WATCH MAYOR ADAMS: "YOU DON'T TAKE OVER OUR BUILDINGS AND PUT ANOTHER FLAG UP"![]()
‘But this is really up to the Food and Drug Administration to make an objective determination.'
Pfizer is in the midst of conducting trials of its two-dose vaccine in children over the age of two.
Pfizer and BioNTech are soon planning to seek approval for their COVID-19 vaccine in children aged five to 11.
WATCH: DOJ SET TO INDICT TEXAS DEMOCRAT REP.HENRY CUELLER, WHAT'S AT STAKE, YOU ASK?![]()
Dr Özlem Türeci, chief physician for BioNTech, announced on German news site Der Spiegel that the companies are set to shortly release results from their study in kids under age 12 and will ask for the shot to be approved for emergency use authorization by the FDA and other agencies.
'In the coming weeks, we will present the results of our study on the five-to-11-year- olds worldwide to the authorities and apply for approval of the vaccine for this age group,' Türeci stated.
She added that the vaccine formula is the same as that approved for adolescents and adults, but that the dose size is smaller.
Currently, the Pfizer vaccine is only approved for children aged 12 and older in both the US and the European Union.
THE HAWAII BILLIONAIRE BATTLE: MEET THE RECLUSIVE RICHEST MAN ON THE ISLAND...![]()
Parents and doctors have been debating about whether or not to inoculate children because they make up 0.1 percent of all Covid deaths in the U.S.
The US government says that it is essential to vaccinate children to slow the spread of COVID-19 and its contagious Delta variant.




 Discover alternative ideas that will make you think
Discover alternative ideas that will make you think Engage in mind bending debate
Engage in mind bending debate Earn points, rise in rank, have fun
Earn points, rise in rank, have fun


