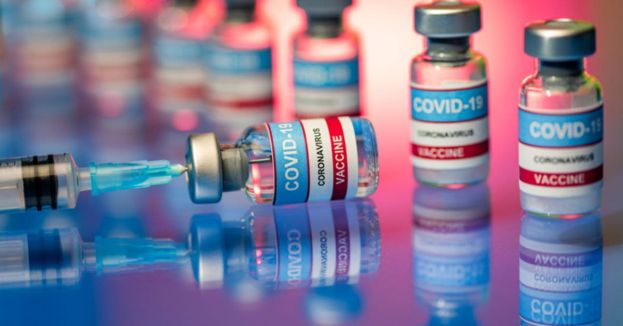
"Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic," acting FDA Commissioner Janet Woodcock said Monday. "Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
UNIVERSITY OF WASHINGTON SET TO ESTABLISH "GAZA CAMP" AFTER BEING DENIED FOR LACK OF LEADERSHIP![]()
The Pfizer shots were previously only approved for use in people 16 and older. Pfizer asked the FDA for use permission in young people after trial results in March found the shots were 100% effective and produced a "robust antibody response." The U.S.-based trial enrolled 2,260 adolescents in the age range. All eighteen of the COVID-19 cases happened in the placebo group, and none transpired within the inoculated group.
YOU WON'T BELIEVE HOW MUCH RESIDENTS IN THESE STATES PAY IN LIFETIME TAXES![]()
"With science guiding our evaluation and decision-making process, the FDA can assure the public and medical community that the available data meet our rigorous standards to support the emergency use of this vaccine in the adolescent population," Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said Monday.
THE ISRAELI RAFAH INVASION PLAN HAS THE WHITE HOUSE IN A TIZZY![]()
“Having a vaccine authorized for a younger population is a critical step in continuing to lessen the immense public health burden caused by the COVID-19 pandemic,” said Marks.
The expanded EUA for the Pfizer shots will open up vaccinations to a broad swath of the people that have been shown less likely than older adults to undergo rigorous disease due to coronavirus infection. Pfizer also announced last week that it expects to file an EUA request for its vaccine to be done in children ages 2 to 11 in September.
THE TRAGIC TALE OF IGNORED WARNINGS: HOW 2023 MAINE MASS SHOOTING COULD HAVE BEEN PREVENTED![]()
"[Sixteen] million more Americans... just became eligible to be vaccinated," Dr. Ashish Jha, dean of Brown University's School of Public Health said Monday. "Vaccinating them will add a lot to population immunity."
U.S. INTELLIGENCE AGENCIES' REPORT RELEASED: WHO WAS RESPONSIBLE FOR NAVALNY'S DEATH?![]()
Till now, approximately 46% of all Americans 16 and older have obtained at least one shot, according to federal data. Vaccinations are higher among adults 18 and older, with roughly 58% having received at least one dose because the other two vaccines from Moderna and Johnson & Johnson have been authorized for adults 18 and older.
WAIT FOR GAS PRICES TO RISE! OVER 30 MISSILES LAUNCHED AT UKRAINE'S ENERGY INFRASTRUCTURE![]()
The FDA has renewed the Fact Sheets for Healthcare Providers Administering the Vaccine (Vaccination Providers) and for Recipients and Caregivers with information to reflect the use of the vaccine in the adolescent population, including the benefits and risks of the Pfizer-BioNTech COVID-19 Vaccine.







 Discover alternative ideas that will make you think
Discover alternative ideas that will make you think Engage in mind bending debate
Engage in mind bending debate Earn points, rise in rank, have fun
Earn points, rise in rank, have fun


