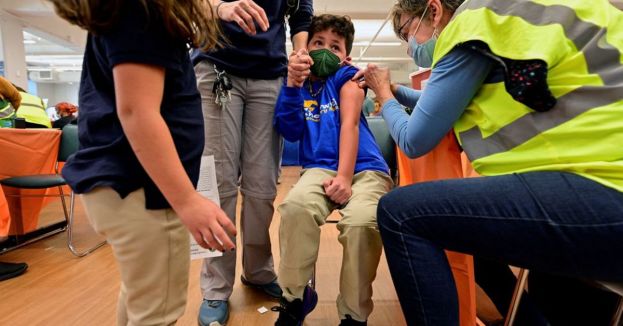
The FDA reviewers said in briefing documents published on Sunday evening that their evaluation did not reveal any new safety concerns related to the use of the vaccine in young children.
The FDA analysis of data from Pfizer’s trial was published ahead of a June 15 meeting of its outside advisers. Recommendations from the external advisers will determine the FDA’s decision on the vaccines.
“Available data support the effectiveness of the Pfizer-BioNTech COVID-19 Vaccine 3-dose primary series in preventing COVID-19 in the age group of 6 months through 4 years,” FDA staff said in the review.
WATCH: "WE WILL OPEN YOU UP LIKE A SOFT PEANUT"![]()
An early analysis of data from Pfizer-BioNTech’s vaccine based on 10 symptomatic COVID-19 cases identified when the Omicron Coronavirus variant was dominant suggested a vaccine efficacy of 80.3% in the under-5 age group.
COVID-19 shots for children under the age of 6 are yet not approved in most parts of the world. It remains unclear how many parents will get their young ones vaccinated as demand has been low for kids aged 5 to 11.
WATCH: HOW WAS JURY SELECTION HANDLED IN TRUMP'S TRIAL?![]()
U.S. President Joe Biden’s Administration expects vaccinations for young children to start as early as June 21 if the FDA and the Centers for Disease Control and Prevention approved the vaccines.
A LOOK INSIDE DEMOCRATS' PLAN TO WIN BACK THE HOUSE WITH RECORD INVESTMENT![]()
Both of the messenger RNA-based COVID-19 vaccines made by Moderna and Pfizer/BioNTech have been related to rare examples of a type of heart inflammation named myocarditis, particularly in young men.
Some nations in Europe have limited the use of Moderna’s shots for younger age groups after some studies revealed that it was connected to a higher risk of heart inflammation.
The FDA announced myocarditis is a known risk associated with the vaccine, yet that the drugmaker’s pediatric trials were not large enough to quantify the frequency of the rare heart inflammation in pediatric age groups.
DEMS' LAST-MINUTE BID TO SAVE SEATS AMID SOUTHERN BORDER TURMOIL!![]()
The Pfizer/BioNTech vaccine is already authorized in the U.S. for people aged 5 and older. The U.S. Centers for Diseases Control and Prevention announced in May that reports of myocarditis after that vaccine had been much lower in 5- to 11-year-old boys than in adolescents and young men, representing just a slightly elevated rate than normal.
ICE BLUNDER: AFGHAN NATIONAL SUSPECTED OF TERROR TIES ESCAPES U.S. TRACKING PROGRAM IN DAYS...![]()
The FDA currently permits Moderna’s vaccine to be used only in adults. Yet some nations enable full-size doses for teens and half-size shots for kids ages 6 to 11 — a step the FDA is considering.
Over 30,000 U.S. children younger than 5 have been hospitalized with COVID-19, and almost 500 Coronavirus deaths have been reported in that age group, according to U.S. health officials.



 Discover alternative ideas that will make you think
Discover alternative ideas that will make you think Engage in mind bending debate
Engage in mind bending debate Earn points, rise in rank, have fun
Earn points, rise in rank, have fun


